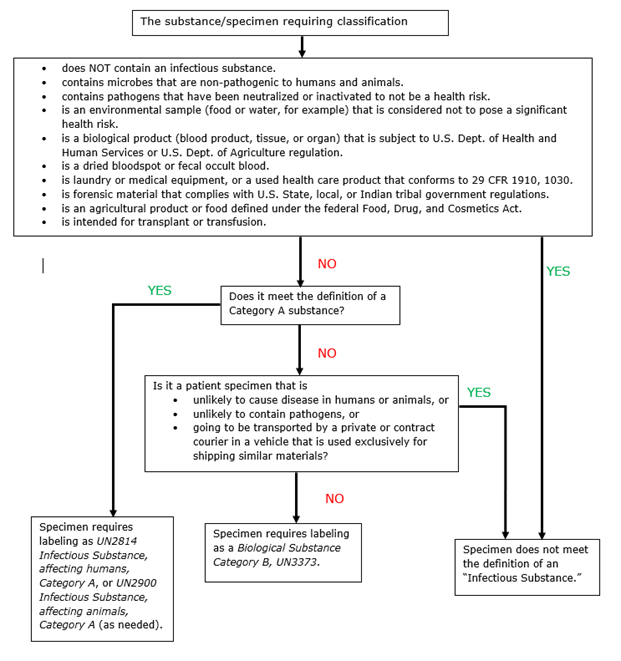
45 exempt human specimen meaning
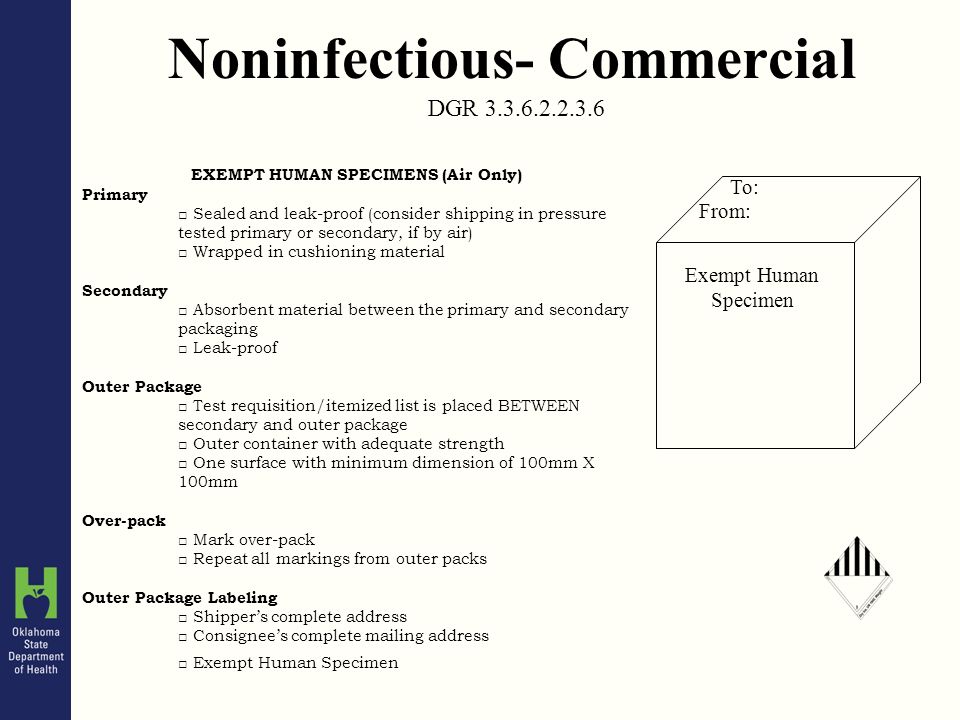
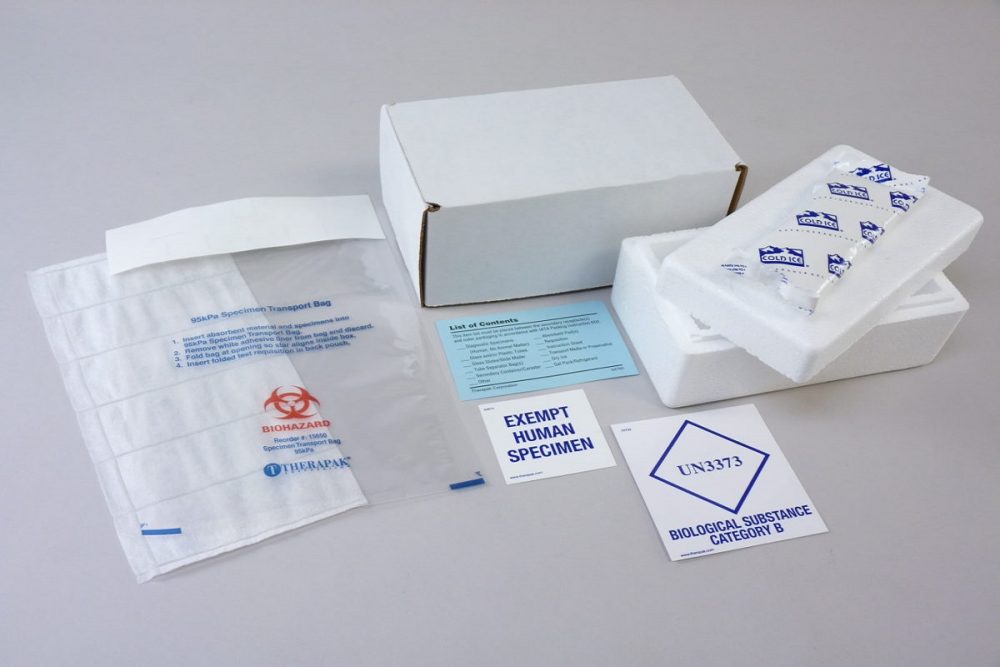
PDF Step 3: Packing Category A and B and Exempt Human and Exempt ... - CDC Step 3: Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid . Use the pages below as a reference for packing Category A, B, and Exempt Specimens. Category A Substance Packaging . NOTE: The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change. IATA Dangerous Goods Regulations | IATA Requirements | Therapak Specimen shipping packages consigned to couriers and air carriers must have the marking "Exempt Human Specimen" and must, at a minimum, meet the following package requirements: 100 mm² dimension on one side. leak-proof primary container. leak-proof secondary container. absorbent between primary and secondary sufficient to absorb total ...
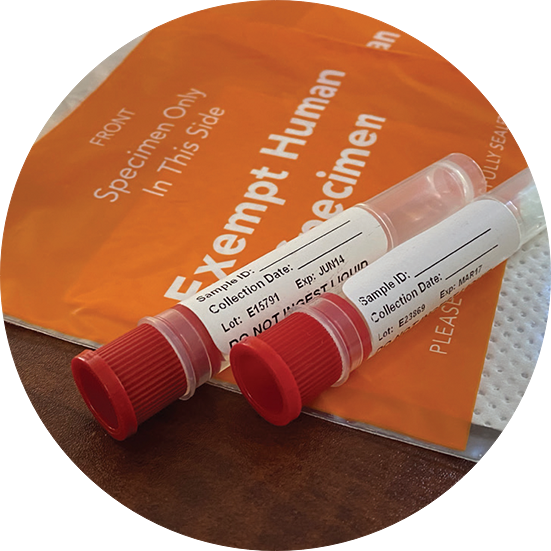
Exempt Animal or Human Specimens | Environment, Health and Safety Patient specimens (containing no other hazardous materials) for which there is minimal likelihood that pathogens are present are not subject to other shipping regulations except: The specimen must be packed in a packaging which will prevent any leakage and which is marked with the words: "Exempt Human Specimen", or. "Exempt Animal Specimen".
Exempt human specimen meaning
What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... Data/Specimen Analysis Research. A study consisting of analyzing data or specimens only, and not involving any interventions or interactions with subjects to collect those data or specimens, is most likely to be exempt if it is not externally funded. Shipping Biological Substances FAQs | UPS - United States Patient specimens for which there is minimal likelihood that pathogens are present are not subject to other provisions of the Regulations provided they are marked with the words "Exempt human specimen" or "Exempt animal specimen" and packaged according to the IATA regulations. (Dangerous Goods Regulations, 3.6.2.2.3.8) PDF Proper Shipment of Patient Specimens and Infectious Substances Definition: Specimens collected from humans or animals including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for ... Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other ...
Exempt human specimen meaning. Guidance for Shipping Biological Materials at Cornell University "Exempt human specimen", "Exempt animal specimen" Not subject to shipping regulations if the specimen is transported in packaging that will prevent leakage and is appropriately marked. Recommend labeling the box with contact information and include itemized list of contents and courtesy letter describing the substances. PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: a. a leak-proof primary receptacle(s); b. a leak-proof secondary packaging; and PDF 1 Meets the definition of human subjects research. specimens if publicly available, or recorded such that subjects cannot be identified* *May be identifiable in limited cases. See §46.104(d)(4)(iii) and (iv) Exemption 5: public service program research or demonstration projects Exemption 6: taste and food quality evaluations Exemption 7: storage of identifiable information or Coded Private Information or Specimens Use in Research, Guidance (2008) Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ...
How to Ship Clinical Samples | FedEx The FedEx ® Clinical Pak may be used to ship any dry clinical sample. Include a marking on the package that properly identifies the shipment as "Exempt Human Specimen" or "Exempt Animal Specimen" as appropriate to comply with current IATA and ICAO regulations. If you prefer, package markings may be in the form of a label. PDF Guidance Doc Infectious Substances - ICAO definition does not include human or animal patient specimens. Medical or clinical wastes are wastes derived from the medical treatment of animals or humans ... If such a packaging is used it must be marked "Exempt human specimen" or "Exempt animal specimen", as appropriate. PDF Regulated and Non-regulated Biological Materials Exempt Human and Animal specimens are considered dangerous goods by IATA until they meet three criteria: 1. A professional determination has been made that the material has a "minimal likelihood:" of containing any pathogens (Need Appropriate Training to meet this criteria) USPS Packaging Instruction 6H | Postal Explorer Marking/Documentation. The outer shipping container must be marked on the address side with the words "Exempt human specimen" or "Exempt animal specimen," as appropriate. In addition, at least one surface of the outer packaging must have a minimum dimension of 3.9 inches by 3.9 inches (100 mm by 100 mm). A shipping paper is not required.
Exemptions (2018 Requirements) | HHS.gov §46.104 Exempt research. (a) Unless otherwise required by law or by department or agency heads, research activities in which the only involvement of human subjects will be in one or more of the categories in paragraph of this section are exempt from the requirements of this policy, except that such activities must comply with the requirements of this section and as specified in each category. Definition of Human Subjects Research | grants.nih.gov Definition of Human Subjects Research. According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or. PDF Guidelines for Human Biospecimen - National Institutes of Health Definition of Human Biospecimens These guidelines apply to human biospecimens , including --but are not limited to --blood and other body fluids, tissues, and other biological material s obtained from humans. Subsets of human materials, such as derived cell lines that are traceable to a human subject or patients Frequently Shipped Biological Material and Proper Classification Professional judgment must be used; if you suspect the specimen may contain an infectious substance, it must be shipped accordingly. These shipments should be packaged using a triple packaging system and marked as "exempt human specimen" or "exempt animal specimen." Exempt patient specimens include: Biopsies. Dried blood spots.
Exempt patient specimens - un3373.it EXEMPT HUMAN SPECIMEN or EXEMPT ANIMAL SPECIMEN. They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required to determine if a substance is exempt under this paragraph. That judgment should be based on the known medical history, symptoms and ...
PDF 3 For the purposes of these Regulations - International Air Transport ... subject to these Regulations unless they meet the criteria (a) The specimen must be packed in a packaging which for inclusion in another class. will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt 3.6.2.2.3.3 Substances in a form that any present patho- animal specimen," as appropriate;
PDF Proper Shipment of Patient Specimens and Infectious Substances Definition: Specimens collected from humans or animals including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for ... Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other ...
Shipping Biological Substances FAQs | UPS - United States Patient specimens for which there is minimal likelihood that pathogens are present are not subject to other provisions of the Regulations provided they are marked with the words "Exempt human specimen" or "Exempt animal specimen" and packaged according to the IATA regulations. (Dangerous Goods Regulations, 3.6.2.2.3.8)
What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... Data/Specimen Analysis Research. A study consisting of analyzing data or specimens only, and not involving any interventions or interactions with subjects to collect those data or specimens, is most likely to be exempt if it is not externally funded.














Post a Comment for "45 exempt human specimen meaning"